By Kim Smiley
The FDA has reported a record number of drug shortages in 2010 that is continuing to increase into 2011. Some of the drugs included in this shortage are chemotherapy drugs. Providers across the U.S. are reporting that the shortages may endanger patients if they are unable to receive the necessary drugs. In some cases, drugs that are more expensive, less effective, or both are being used. Or, patients are turning to the “grey market”, buying drugs of questionable safety that have, in most cases, been significantly marked up. Additionally, because already approved drugs are needed for clinical trials, some clinical trials have been delayed, limiting the addition of new medications.
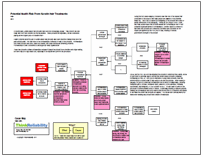
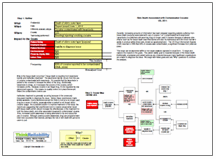
We can look at this issue in a Cause Map, a visual root cause analysis. A Cause Map connects the impacts to the goals of an organization, or in this case, the U.S. healthcare system. The patient safety goal is impacted because of the risk to patient health. The organization goal is impacted because of delayed clinical trials. The patient services goal is impacted because of the lack of needed medication. Also, the property goal is impacted because of the replacement with more expensive medications. We use these goals as the basis for our Cause Map, then ask “Why” questions to complete the analysis.
 Insufficient supply is caused by demand greater than supply. Both of these factors can contribute to the overall effect. Although there are several reasons for increased demand, the most pertinent to this issue appears to be hoarding – where physicians hear of shortages and are attempting to create a stockpile for their patients. However, increased insurance coverage and general increased need for medication for diseases such as cancer are also likely contributing.
Insufficient supply is caused by demand greater than supply. Both of these factors can contribute to the overall effect. Although there are several reasons for increased demand, the most pertinent to this issue appears to be hoarding – where physicians hear of shortages and are attempting to create a stockpile for their patients. However, increased insurance coverage and general increased need for medication for diseases such as cancer are also likely contributing.
Reduced supply is also contributing to the shortage. Shortage of raw ingredients is considered to account for about 10% of the issue, with quality issues and insufficient production accounting for the rest. In some cases, manufacturers are believed to be limiting their production – or ending it all together – because the drugs do not provide much profit. Many of the limited drugs are generics, which do not provide as much profit as name brand drugs, especially as drug profits were limited by the Medicare Prescription Drug, Improvement and Modernization Act of 2003, which limited price increases in an attempt to limit Medicare spending. The resulting drug shortage – which is sometimes resulting in paying for much more expensive drugs – is certainly an unintended consequence of this act.
Despite best intentions, changes made to fix an identified problem may in fact cause another one – sometimes one that is far greater. This is why follow-up to implemented solutions must occur at regular intervals, and must include a comprehensive assessment of whether the solutions are effective.
Some of the solutions recommended to prevent the issues caused by these drug shortages are to create a national stockpile of certain kinds of drugs and requiring notification to the FDA of supply shortages. In the meantime, the FDA is providing guidance to patients and providers to attempt to ease the ongoing issues.